Practice Management Corner
Practice Resources

As part of our ongoing member support, we are introducing a new website feature to help with common practice management challenges. We welcome your input with questions or challenges you would like to address. Please send them to [email protected].
For this blog we are addressing questions around transition immunotherapy due to supply chain challenges, pricing, and more.
Latest Blog Post
Hot sessions generating great questions from the 2024 Annual Meeting included Dr. Mason’s session on drug-induced sleep endoscopies (DISE). Below are the most common. Read More
FAQS and Myths Around Transitioning Immunotherapy
- Do I need to re-test my all my patients?
- This is not necessary, but certainly an option and physician discretion. The AAOA does, however, recommend vial testing prior to introducing any new vial, as with a new lot from the same manufacturer.
- Do I need to keep two vendors as I transition to a new source as I enroll new immunotherapy patients?
- As with question #1, a vial test will help you determine safe dosing.
You could opt to keep existing patients or those close to completion of immunotherapy on your existing vendor while you switch new immunotherapy patients to the new product.
- Do I need to keep existing patients on their current antigen vendor and batch?
- You can certainly keep your existing patients on the current antigen product, but you do not have to.
- Is there a dose adjustment during manufacturer transition, if so, how is this achieved?
- Due to potency fluctuations between manufacturers, reducing the patient’s last injection by at least 50% should strongly be considered. For example, if the patient’s last injection was 0.5mL, the patient would receive 0.25mL (from same dilution if applicable), with new vial formulation. The AAOA also strongly recommends vial testing during manufacturer transition, similar as when switching lot to lot using same manufacturer.
- My current w/v is different than what my new antigen vendor is offering, how do I adjust my dosing?
- 1:40w/v versus a 1:20w/v = cutting the formulary dose in half. For example, you were placing 1.0mL 1:40w/v concentration in formulary, you would insert 0.5mL 1:20w/v concentration.
- 1:20w/v versus a 1:10w/v = cutting the formulary dose in half. For example, you were placing 1.0mL 1:20w/v concentration in formulary, you would insert 0.5mL 1:10w/v concentration.
- 1:10w/v versus 1:20w/v= double the mL in formulary dose. For example, you were using 0.5mL 1:10w/v concentration in formulary, you would insert 1.0mL 1:20w/v concentration.
- You may also consider diluting the w/v extract to previous concentration (if applicable), to avoid adjusting the vial mix and further manipulating the allergen components of the mix.
This can be achieved by taking the desired vial size and dividing by 2.
- Example- You would like to dilute 1:20w/v extract to 1:40w/v using 50mL sterile empty vial. 50/2= 25mL
- Insert 25mL 1:20w/v and 25mL 50% Glycerin= 50mL 1:40w/v extract.
- Is there any concern about varying Species identification between the manufacturers?
- All manufacturers formulate extracts using the same species. There are a few exceptions. However, if the species nomenclature is not exact, the cross-reactive alternative is offered as a substitute. With dose-reduction considerations discussed above and performing new vial testing with each transition, this accommodates potency differences when using a cross-reactive substitution. Climate has a huge impact on production, so it is not atypical for a species to backorder due raw material shortages. Often, physicians will look to purchase a cross-reactive alternative at that time. The safest protocol is to perform skin testing with the substitute species or, at minimum, perform a vial test with the new vial containing the new cross-reactive species.
- What should we be logging? (Can pull in the USP details for answer here, if question appropriate)
7. What is the best approach to restarting subcutaneous immunotherapy (SCIT) after a prolonged absence?
- Re-starting immunotherapy after a prolonged absence without dose adjustment can increase the risk of a serious systemic adverse event. It is important to consider the patient’s phase of immunotherapy — maintenance versus escalation. Any changes in immunotherapy due to changes in the constituents of immunotherapy, including changes in production lot, manufacturer, or extract type (aqueous, glycerinated, standardized, non-standardized) also put the patient at increased risk of an adverse event. Safety is paramount when re-starting immunotherapy injections.
- Retesting to determine the safe starting dose is the most conservative approach.
- Reducing the dose of the new antigen is another approach to allow for gradual and potentially safer introduction of the newly sourced antigen.
- The use of vial testing for the first injection provides an additional level of safety and is recommended.
When determining an appropriate dose for restarting or making antigen adjustments during immunotherapy, the clinical features of each individual patient are essential, including the severity of allergic disease, prior systemic reaction, time of year for pollen allergies, health status including medications, and asthma presence, severity and control. If the patient is at increased risk of adverse reaction due to any of the above factors, decrease in dosing is recommended at the first injection. The escalation schedule will similarly depend on individual factors and tolerance of prior injections. Patients who are in the Maintenance Phase of SCIT may be able to tolerate higher initial dosing and more rapid new antigen escalation schedules than those patients in the Escalation Phase of all allergens during SCIT.
Below is a sample dosing schedule.
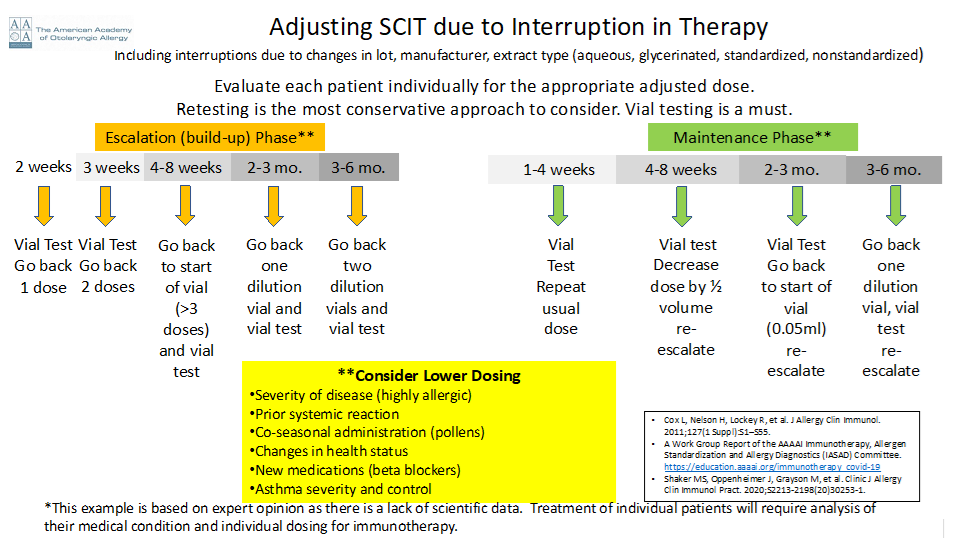
Download the pdf of the QA Focus Tips for Transitioning Immunotherapy here.
___________
References: Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter, third update. Journal of Allergy and Clinical Immunology, January 2011; Volume 127 (1), Supplement, page S1-S55.
Important Information
- Online Sterile Allergen Extract Compounding Module
- Special AAOA member pricing for Valiteq Media Fill Test kits
- Special AAOA member pricing for Valiteq Gloved Finger Test kits
- Practice Resource Tool Kit
- Clinical Care Statement
- USP 797 Workshop Handout
- AAOA YouTube Video
- Valiteq Order Form
- Valiteq Kit Instructions
Recent Updates
Modifier 25: Significant & Separately Identifiable
Practice Corner FAQ’s https://youtu.be/F7rlAIK27bc We receive regular questions about when to use modifiers. The video clip is an excerpt from our Annual Meeting 2024 presentation,…
Drug-Induced Sleep Endoscopies (DISE): Questions Answered
Hot sessions generating great questions from the 2024 Annual Meeting included Dr. Mason’s session on drug-induced sleep endoscopies (DISE). Below are the most common. Q: Who…
Navigating Changes in Payer Policies
January is the ideal time to do a payer policy review. Many payers make changes and updates effective at the start of the year. Below…
CMS Releases Updated Guidance for Using G2211: Complex Patient Care Add-on Code
Beginning January 1, the Centers for Medicare & Medicaid Services (CMS) expanded its billing policy for HCPCS code G2211 (Visit complexity inherent to evaluation and…
Allergy Testing Medicare Definitions & Coverage
Practice Corner FAQ’s Q: Is there a maximum number for allergen tests? A: For an initial diagnostic evaluation, the Centers for Medicare & Medicaid Services…
New guidance for payers related to unreasonable documentation for immunotherapy
Newly published guidance from the American College of Allergy, Asthma & Immunology (ACAAI), the American Academy of Allergy, Asthma & Immunology (AAAAI) and the American…
Expanding Access through the ENT PA Model, Same-Day Appointments and Walk-In Urgent Care
By Gavin Setzen, MD, FACS, FAAOA, FARS, and Drew Franklin, MBA, FACMPE The healthcare landscape is evolving rapidly, with a pressing need to expand patient…







