“I owe a lot to the AAOA and the fellowship process. Obtaining that level of expertise improved my patient outcomes greatly and made me and my practice more marketable. I was asked about my allergy practice/fellowship at several job interviews…
Allergenic Extracts
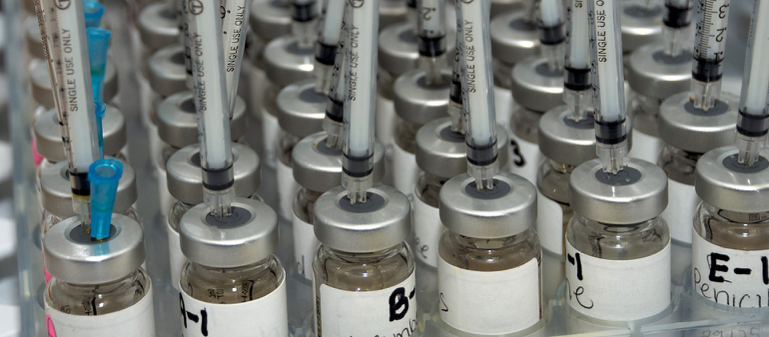
In late November 2013, Congress passed the Compounding Bill. This legislation enforces regulation of compounding pharmacies, as a result of injections of contaminated compounded drugs – which caused 64 deaths and many more nonfatal injuries.
PLEASE NOTE: The preparation of allergenic extract vials is considered compounding. The AAOA is pleased that the new legislation does not restrict or limit the ability of Allergists to compound allergy vaccines. AAOA members may have to adopt additional measures to ensure sterility, based on USP guidelines, but fortunately the ability to continue compounding extracts remains.
The statute contains two provisions that may impact allergy immunotherapy. Since the law is effective, it is recommend that you implement these changes as soon as possible. The AAOA expects practices will be subject to inspections to monitor compliance.
The first requirement is that all compounded sterile preparations have a prescription. This should not cause much of a problem since most AAOA members already have an order sheet of some sort, which is used to instruct staff to make a new or renewal vial for a patient. These order sheets can be labeled at the top as a prescription for a specific patient, and they can have a line at the bottom for the physician’s (or other appropriately licensed provider’s) signature. Again, you are already signing off on this process, as well as the shots, so this should not be a huge practice change.
In addition, it appears that physicians will be required to comply with all of the USP 797 sterile compounding rules. Fortunately, because of work by the AAOA in conjunction with JCAAI back in 2006, USP 797 contains specific rules for allergy vaccine compounding that are much less stringent than the rules applicable to other compounded sterile products. While the USP rules are not draconian (no filtered air or laminar flow hoods required), they do increase the standard for sterility.You can read the relevant Allergy Section of USP 797 by clicking here or read the rules below.
“Allergen extracts, as compounded sterile preparations (CSPs), are single-dose and multiple dose intradermal or subcutaneous injections that are prepared by specially trained physicians and personnel under their direct supervision. Allergen extract, as CSPs, is not subject to the personnel environmental and storage requirements for all CSP microbial contamination risk levels in this chapter, BUT only if all of the following criteria are met:
- The compounding process involves simple transfer via sterile needles and syringes of commercial sterile allergen products and appropriate sterile added substances (e.g., Glycerin, phenol in sodium chloride injection).
- All allergen extract as CSPs shall contain appropriate substances in effective concentrations to prevent the growth of microorganisms. Non-preserved allergen extracts shall comply with the appropriate CSP risk level requirements in the chapter.
- Before beginning compounding activities, personnel perform a thorough hand cleansing procedure by removing debris from under fingernails using a nail cleaner under running warm water followed by vigorous hand and arm washing to the elbows for at least 30 seconds – with either non-antimicrobial or antimicrobial soap and water.
- Compounding personnel don hair covers, facial hair covers, gowns and face masks.
- Compounding personnel perform antiseptic hand cleansing with an alcohol – based surgical hand scrub with persistent activity.
- Compounding personnel don powder – free sterile gloves that are compatible with sterile 70% isopropyl alcohol (IPA) before beginning compounding manipulations.
- Compounding personnel disinfect their gloves intermittently with sterile 70% IPA when preparing multiple allergen extracts as CSPs.
- Ampule necks and vial stoppers on packages of manufactured sterile ingredients are disinfected by careful wiping with sterile 70% IPA swabs to ensure that the critical sites are wet for at least 10 seconds and allowed to dry before they are used to compound allergen extracts as CSPs.
- The aseptic compounding manipulations minimize direct contact contamination (e.g., from glove, fingertips, blood, nasal and oral secretions, shed skin and cosmetics, other non-sterile materials) of critical sites (e.g., needles, open ampules, vial stoppers).
- The label of each multiple-dose vial (MDV) of allergen extracts (as CSPs) lists the name of one specific patient, a “by use date” (BUD) and storage temperature range that is assigned based on manufacturers recommendations or peer-reviewed publications.
- Single-dose allergen extracts as CSPs shall not be stored for subsequent additional use.
Personnel who compound allergen extracts as CSPs, must be aware of greater potential risk of microbial and foreign material contamination when allergen extracts are compounded in compliance with the foregoing criteria instead of the more rigorous standards in the USP chapter for CSP microbial contamination risk levels. Although contaminated allergen extracts as CSPs can pose health risks to patients when they are injected intradermally or subcutaneously, these risks are substantially greater if the extract is inadvertently injected intravenously.”
References
1. Lay PC, Bass R, Lin SY. Allergen vial mixing and immunotherapy: risks of infection and vial contamination. Otolaryngol Head Neck Surg. 2007 Aug;137(2):243-5
2. Lin SY, Lay PC, Hughes LF, Bass R. The safety of multi-dose vials in allergy immunotherapy. Otolaryngol Head Neck Surg. 2008 Aug;139(2):195-7
3. Lay PC, Bass R, Hughes LF, Lin SY. Risks of allergy vial contamination: Comparison of mixing in-office versus under ventilation hood. Otolaryngol Head Neck Surg. 2008 Sep;139(3):364-6.
4. Gilbert KC, Sundareshan V, Bass RM, Lin SY. Antibacterial Properties of Additives Used in Injection Immunotherapy. Int Forum Allergy Rhinol. 2011 Dec 7. doi: 10.1002/alr.20105. [Epub ahead of print].